Smart BOM (Bill of Material) validations Agent
- Sarb Randhawa

Late Compliance Turns Into Costly Business Risk
When compliance problems show up at the end of the product cycle, it’s never just a paperwork issue—it’s a business issue. Teams spend weeks validating Bills of Materials, only to discover late that a component is obsolete, a label is missing data, or a material no longer meets today’s rules. In medical devices, tighter MDR timelines and UDI requirements mean even a small mismatch can derail certification and push out revenue. In pharma, the revised Annex 1 expects traceability and control across people, materials, and equipment—so gaps in supplier evidence or component records become deviations or batch holds. What leaders see: slip pages on launch timing, higher rework costs, and tougher audits. What teams feel: constant firefighting instead of focused execution.
The pattern is consistent and structural, not personal. Most companies break in the same two places. First, critical evidence lives in silos—PLM, ERP, QMS, spreadsheets—so the “single source of truth” for part status lags reality. Second, processes aren’t built for today’s speed of regulatory change and supply volatility, so risks surface late when fixes are slow and expensive. Stress concentrates in sourcing and quality: sourcing absorbs supplier churn and compliance switches at premium costs; quality faces escalating scrutiny on documentation, traceability, and monitoring. Planning, production, and logistics inherit the shock through reschedules, rework, and missed windows. The leadership takeaway: bring compliance data forward in the cycle, simplify handoffs, and anchor decisions to a shared record—so your organization spends less time reacting and more time delivering.
Why End-of-Line BOM Errors Hurt Performance
When product teams only discover non-compliant or obsolete parts at the finish line, everyone pays the price—engineers scramble to redesign, buyers chase scarce alternatives, quality prepares for tough questions, and leaders watch timelines slip. In electronics, even a small miss against RoHS/REACH can stall shipments or force a market withdrawal. In medtech, a missing UDI attribute or an outdated declaration can trigger documentation rework just when a notified body review is due. In pharma, Annex 1 raises the bar on traceability and supplier control, so incomplete linkages between materials, components, and records quickly turn into deviations or batch holds. None of this is about one team underperforming; it’s a system set up to catch issues late—when fixes are slow and expensive.
Most companies break in the same two places. First, supplier evidence and change control live in too many silos—PLM, ERP, QMS, spreadsheets—so the “truth” about a part’s compliance status lags reality. Second, processes haven’t kept pace with fast-moving rules and supply swings, so good people follow workflows that can’t see risks early. The result is stress where it hurts most: sourcing and quality. Sourcing absorbs supply shocks and compliance churn, often at premium cost. Quality faces tighter scrutiny on documentation, traceability, and real-time controls. Planning, production, and logistics then inherit the fallout through reschedules, rework, and missed windows. The fix starts with earlier visibility, cleaner handoffs, and a single source of record—so teams can focus on building, not firefighting.
When BOM Gaps Trigger Costly Compliance Chaos: Shift Validation Left for Audit-Ready Releases
Two weeks from launch, a small data gap surfaces in a Bill of Materials—and the date moves. What reads like an SOP step on paper becomes a leadership problem in practice: engineers rush to redesign, sourcing hunts for compliant alternates, quality braces for tougher scrutiny, and the P&L absorbs delay costs. In medical devices, MDR’s tighter documentation and UDI traceability mean even minor mismatches can cascade into certification slippage and missed revenue windows. In pharma, the revised Annex 1 expects a living contamination control strategy and real time monitoring; weak linkages between components, suppliers, and records quickly translate into deviations or batch holds. Electronics faces its own pressure cooker: RoHS/REACH enforcement has intensified, with recalls, fines, and lost market access when restricted substances or documentation gaps are found late.
The fix isn’t heroics; it’s design in assurance. Validation must shift left—baked into SOPs for design and change, not staged at release. A single source of truth connects PLM, ERP, and QMS so everyone sees the same part status, supplier evidence, and regulatory fields in real time. An AI/alerting layer watches for obsolete parts, RoHS/REACH updates, UDI data issues, supplier notices, and long lead times—then proposes compliant, available alternatives with cost and schedule impact. That’s how releases become audit ready by design, not a last minute scramble, and how teams spend more time building and less time firefighting. For leaders, the payoff is tangible: fewer slips, cleaner audits, steadier revenue recognition, and resilience when rules and supply shift again.
What InspireXT’s Smart BOM Validation AI Agent actually delivers?
- What you get, tangibly
- Smart BOM Validation agent embedded in design and change: automatic checks for lifecycle status, RoHS/REACH, UDI fields, country rules, AML/AVL, and approved alternates—at the point of work, not at release.
- “Shift left” SOP validation: mandatory, auditable gates inside design and change workflows with clear owners, captured approvals, and revalidation-on-demand.
- Risk-to-action recommendations: compliant alternates with lead time, cost, and supplier impact; flags for long-lead or end of life risk; alerts tied to real parts and real dates.
- Single Product Record: one view of item/BOM status, supplier evidence, and change history for engineering, sourcing, quality, and manufacturing.
- What’s repeatable vs. tailored
- Already built: accelerators for lifecycle/compliance checks, standard data models (item, AML, BOM, CO), prebuilt workflows and alerts, dashboards for readiness/audit.
- Tailored per client: rules by region/industry (e.g., UDI fields, Annex 1 evidence links), sourcing policies, exception thresholds, supplier data onboarding, and KPI definitions.
- Systems we connect today
- PLM as product/system of record; ERP/SCM for cost, supplier, and lead times; QMS for CAPA/audit evidence; MES for effectivity and execution; WMS for readiness; CRM/Sales for launch readiness signals; data/alerting layer for real‑time nudges.
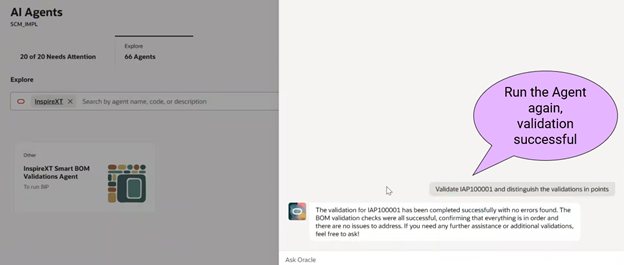
Design Engineer runs the AI agent and provide Change order number for validation
- Data and audit reassurance
- Every check, decision, and approval is time‑stamped, role‑based, and traceable back to the item/BOM and change—so releases are audit‑ready by design, and evidence is one click away.
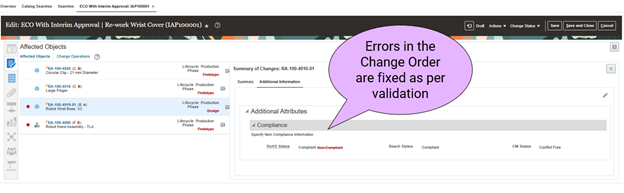
- Correct the validation errors
- Run the Agent once again to confirm validation errors are fixed
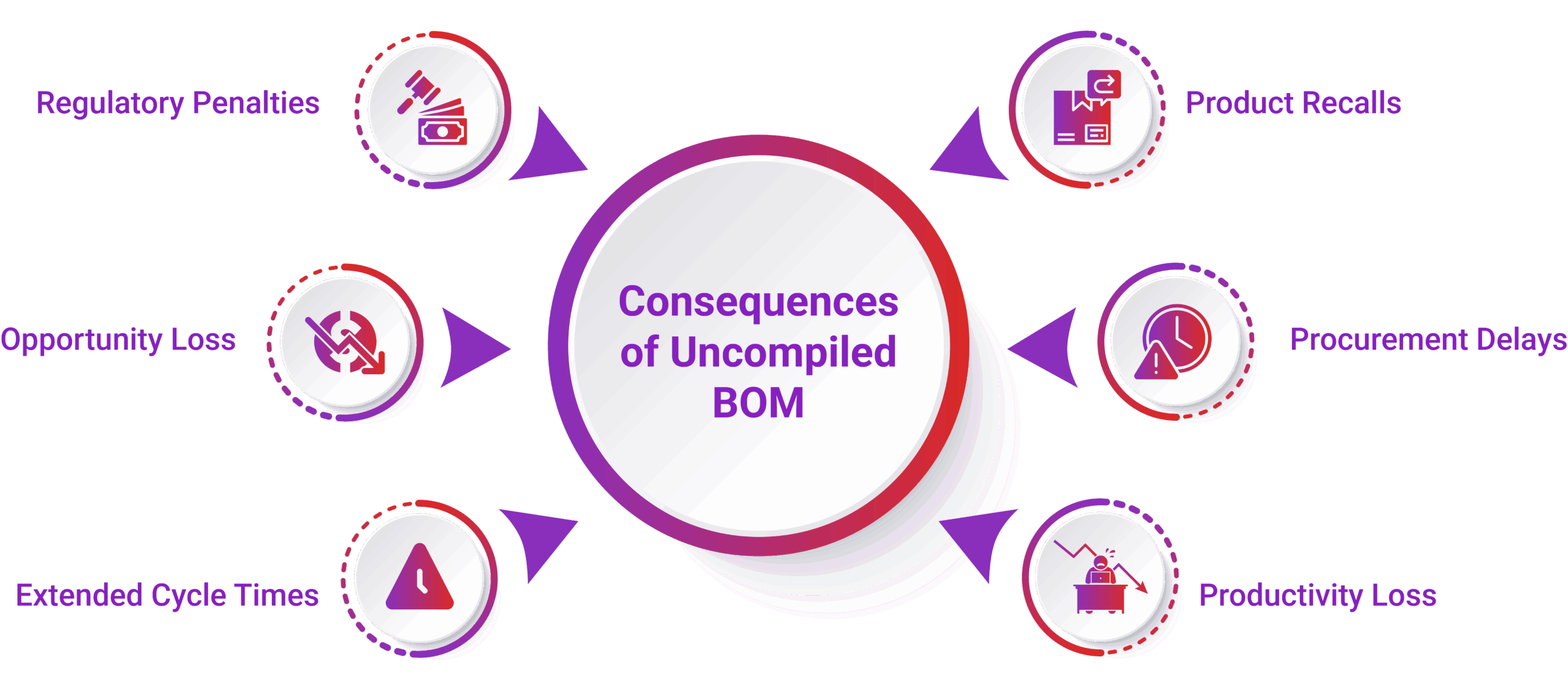
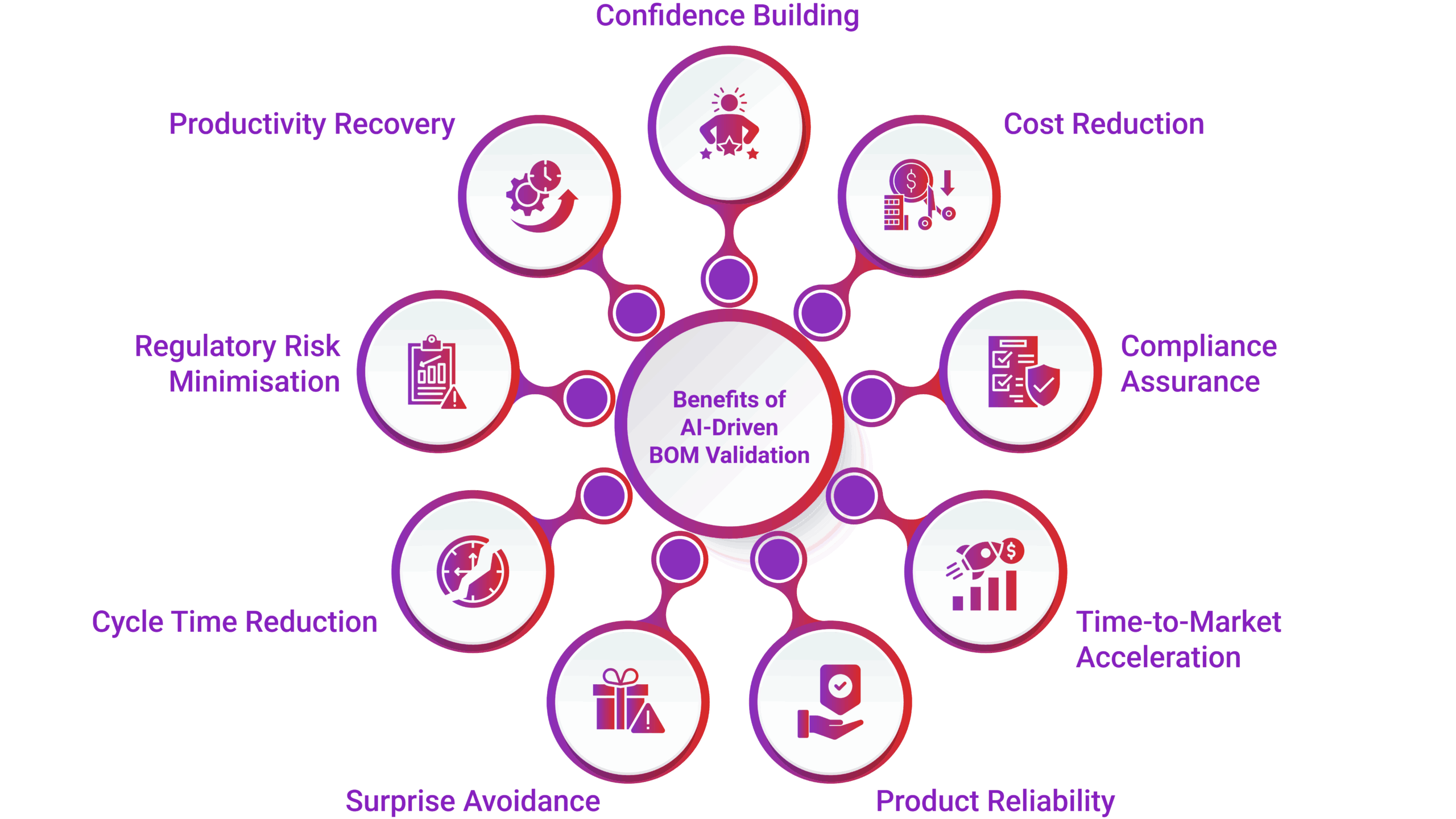
Key Benefits of Smart BOM Validation with AI Agents
- Reduce costly rework and delays
Catch issues early to avoid expensive redesigns and production stoppages. - Ensure compliance “by design”
Embed regulatory checks (e.g., RoHS, REACH) directly into the design process. - Accelerate time-to-market
Streamline validation and change control to launch products faster. - Build reliable and sustainable products
Use up-to-date, compliant components that support long-term product integrity. - Avoid late-stage surprises and redesigns
Proactively identify lifecycle and compliance risks before manufacturing. - Reduce cycle times and procurement delays
Enable faster sourcing decisions with validated, approved components. - Minimize regulatory risks and fines
Stay ahead of global compliance requirements to avoid penalties. - Recover productivity losses
Free up engineering and procurement teams from manual, reactive tasks. - Gain confidence across the value chain
Improve predictability in planning, sourcing, production, and service.
Smart BOM Validation AI Agent Enhancements
As industries worldwide move toward smarter, faster, and more compliant product development, it is imperative to adopt automated validation mechanisms before products reach the manufacturing stage. InspireXT is committed to leading this transformation.
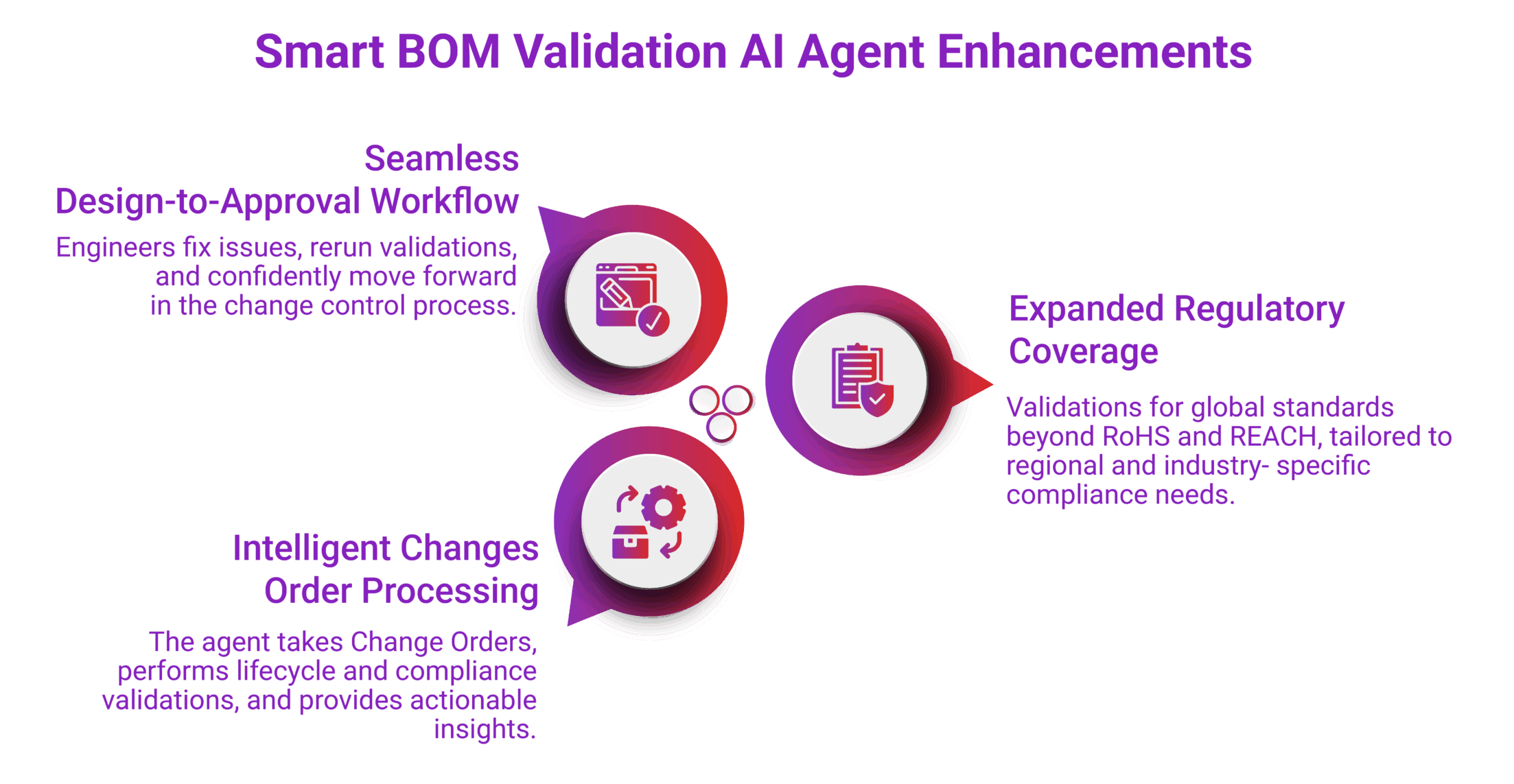
We are actively enhancing our Smart BOM Validation AI Agent to support:
- Expanded Regulatory Coverage
Incorporating validations for additional global standards beyond RoHS and REACH, tailored to regional and industry-specific compliance needs. - Intelligent Change Order Processing
The agent will continue to take Change Orders as input, perform lifecycle and compliance validations, and provide actionable insights to design engineers. - Seamless Design-to-Approval Workflow
Engineers will be able to fix issues, re-run validations, and confidently move forward in the change control process—all within a few clicks.
These enhancements will help organizations avoid late-stage surprises, accelerate time-to-market, and ensure compliance by design, ultimately building reliable, sustainable, and regulation-ready products.
Request for
services
Find out more about how we can help your organisation navigate its next. Let us know your areas of interest so that we can serve you better.


